Catalytic alkylation of unactivated C(sp3)–H bonds for C(sp3)–C(sp3) bond formation
Abstract
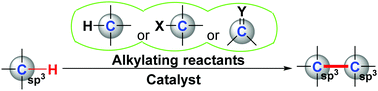
The construction of carbon–carbon bonds is a central tenet of modern synthetic chemistry. Metal-catalyzed C–H functionalization can serve as a particularly powerful tool for achieving carbon–carbon bond formation. This review summarizes the early adventures and recent advancements in catalytic transformation of unactivated C(sp3)–H bonds into C(sp3)–C(sp3) bonds. To date, three main strategies have emerged to enable this transformation, namely, metallocarbene-triggered C(sp3)–H alkylation, auxiliary-directed C(sp3)–H alkylation, and photo-induced C(sp3)–H alkylation. Compared with traditional cross-coupling reactions having both coupling partners activated with functional groups or base-promoted enolate chemistry, catalytic alkylation of unactivated C(sp3)–H bonds for C(sp3)–C(sp3) bond formation not only offers novel disconnections in retrosynthetic analysis, but also represents the future trend in green and atom-economic chemistry.


 Please wait while we load your content...
Please wait while we load your content...