Minimization of solid-state conformational disorder in donor–acceptor TADF compounds†
Abstract
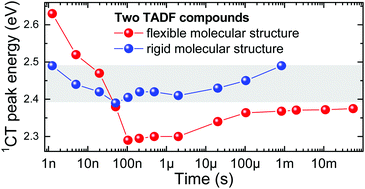
Thermally activated delayed fluorescence (TADF) compounds with a flexible donor–acceptor structure suffer from conformational disorder in solid-state, which deteriorates their emission properties as well as OLED performance. Accordingly, TADF materials with predictable solid-state emission properties are highly desirable. In this work, we analyse the relation between the molecular rigidity and solid-state TADF properties. Two TADF compounds, 4,6-bis(2-methyl-4-(10H-phenothiazin-10-yl)phenyl)pyrimidine (PTZ-mPYR) and 1,2,3,4-tetrakis(carbazol-9-yl)-5,6-dicyanobenzene (4CzPN), with similar emission properties in toluene solutions but different rigidity of the molecular structure were systematically studied. The analysis was supplemented by comparison of solid-state TADF properties of PTZ-mPYR with its analogue 4,6-bis(4-(10H-phenoxazin-10-yl)phenyl)pyrimidine (PXZ-PYR), bearing a more sterically constrained planar electron-donor unit. All compounds showed conformational disorder in diluted polymer films; however its extent directly depended on the molecular structure. Large dispersion of singlet–triplet energy gaps resulted in remarkably prolonged TADF lifetime for PTZ-mPYR with a less sterically constrained donor unit. In contrast, weakened conformational disorder in rigid 4CzPN with sterically crowded donor units was shown to ensure rapid TADF decay with only a threefold lower solid-state rISC rate as compared to toluene. Similarly, selection of a more sterically constrained planar electron-donor unit was also shown to be preferable for lowering the conformational disorder. Our findings are important for the future design of compounds with efficient solid-state TADF as well as for the further application in OLEDs with low external quantum efficiency roll-off.


 Please wait while we load your content...
Please wait while we load your content...