Interactions between alloy elements and oxygen at the steel–liquid LBE interface determined from first-principles molecular dynamics simulations
Abstract
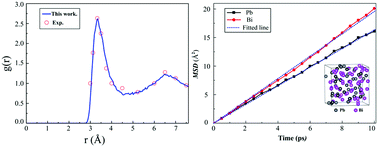
Lead bismuth eutectic (LBE) alloy shows high potential for application in advanced nuclear systems such as lead-alloy-cooled fast reactors. However, high-temperature LBE liquid is prone to corrode the reference containment material, typically made of steel, through a process known as liquid metal corrosion. In this work, an extensive set of first-principles calculations was performed to investigate the diffusion behavior of steel alloy elements and O in liquid LBE. The results showed Bi atoms diffusing a little bit faster than Pb atoms, and the Ni atoms in steel being most likely to dissolve into the LBE. Compared to Cr atoms, Fe atoms were calculated to diffuse more slowly, and Ni atoms more rapidly. In the presence of Al and/or Si, Al–O and Si–O pairs were calculated to be more stable than Fe–O/Cr–O pairs and to be inclined to form protective stable Al/Si related oxides. The Ni–O distance and pair formation energy in LBE indicated the Ni–O pair to be inclined to decompose over a period of time. We expect these data to be used as indispensable information for understanding the dissolution and oxidation corrosion behavior of steels in liquid LBE.


 Please wait while we load your content...
Please wait while we load your content...