Defining aluminum-zoning during synthesis of ZSM-5 zeolites†
Abstract
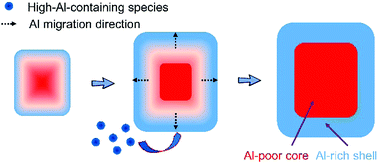
ZSM-5 zeolites attract considerable attention owing to their wide range of applications in catalysis and separation. The crystals that are synthesized with tetrapropylammonium ions (TPA+) as the template show aluminum-zoning, i.e. aluminum being concentrated in the rim part of the crystal. Here, we study the aluminum distribution within individual crystals as a function of synthesis time and find that the degree of aluminum-zoning evolves. Crystals with inhomogeneous aluminum distribution persist since their emergence from the early stages of hydrothermal treatment. The degree of aluminum-zoning in the crystals increases with the synthesis time, accompanied by an increase in the crystal size and subsequently the formation of a well-defined crystal morphology. This indicates a gradual aluminum migration toward the crystal surface during the course of crystallization. Moreover, the addition of high-aluminum-containing species to the existing crystals preferentially takes place at the late stages of synthesis, which contributes to the inhomogeneous aluminum distribution within a crystal. As a result, the finally formed crystals have not only the largest crystal size but also the highest degree of aluminum-zoning. The insight into the origin of aluminum-zoning that our work provides advances our understanding of the relationship between aluminum distribution in zeolites and the synthesis time to design better catalysts.


 Please wait while we load your content...
Please wait while we load your content...