Quantum chemical calculations on NbO and its reaction with methane: ground and excited electronic states†
Abstract
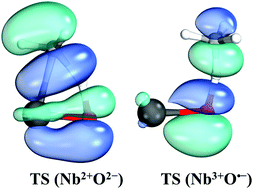
Multi-reference configuration interaction and coupled cluster calculations were performed in conjunction with large basis sets for neutral NbO. We report accurate bond lengths, harmonic vibrational frequencies, anharmonicities, excitation energies, dipole moments, binding energies, and spin–orbit splittings, for the ground state 4Σ− and several excited electronic states. Potential energy curves were constructed for both the anion and neutral species. The potential energy curves for NbO− revealed its ground state as 3Σ−, the same as for NbO+. The calculated ionization energies and electron affinities are in excellent agreement with experiment. Bonding patterns were proposed for several electronic states. Doublet and quartet states are better described as Nb2+O2− with a closed shell oxygen terminal and a niobium center with three localized electrons. The reaction of these states with CH4 occurs via a [2+2] mechanism and leads to a highly endothermic reaction with large activation energy barriers. On the other hand, the lowest sextet state of NbO (Nb3+O˙−) can produce methanol through a radical mechanism with small energy barriers.
- This article is part of the themed collection: 2019 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...