Molecular dynamics simulations reveal how graphene oxide stabilizes and activates lipase in an anhydrous gas†
Abstract
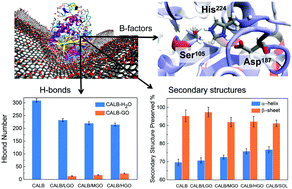
The interaction between Candida antarctica lipase B (CALB) and graphene oxide (GO) in an anhydrous gas was studied using molecular dynamics (MD) simulations augmented with a simulated annealing procedure to accelerate relaxation toward equilibrium. Three kinds of GO sheets with different oxygen contents were constructed to elucidate their effectiveness for stabilizing the active CALB conformation. It was shown that electrostatic forces are pivotal for the formation of CALB/GO complexes, and that a GO sheet with a higher oxygen content leads to stronger association with the protein. The simulation results suggest replacement of protein-binding water molecules by the GO surface, which was confirmed by thermogravimetric analysis. The CALB/GO assembly stabilizes the active enzyme conformation at elevated temperatures and, moreover, increases the protein flexibility near its active sites. The molecular details of GO interaction with CALB and the consequential effects on CALB stability and functionality are important for the development of unprecedented applications of gaseous enzymatic catalysis.


 Please wait while we load your content...
Please wait while we load your content...