Dependence of deposition method on the molecular structure and stability of organosilanes revealed from degrafting by tetrabutylammonium fluoride†
Abstract
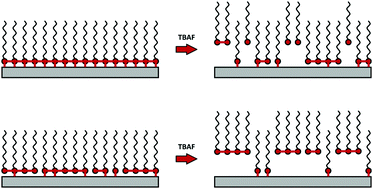
We probe the structure of self-assembled monolayers (SAMs) comprising organosilanes deposited on flat silica-based surfaces prepared by liquid and vapor deposition by removing the organosilane molecules gradually from the underlying substrate via tetrabutylammonium fluoride (TBAF). Removal of organosilanes from the surface involves the cleavage of all pertinent Si–O bonds that anchor the organosilane molecules to the SAM, i.e., direct organosilane-surface linkages and in-plane crosslinks between neighboring organosilanes. We gain insight into the organosilane structure and stability by monitoring the organosilane density as a function of exposure time to TBAF. Degrafting of trifunctional chloro- and methoxy-alkylsilanes deposited from solution yields similar degrafting kinetics. We observe fast degrafting for organosilane SAMs deposited from the vapor phase, indicating that SAMs prepared in this manner form more loosely packed arrays, with less in-plane connectivity, compared to their solution-deposited counterparts. Bulkier, fluorinated silanes form more stable SAMs due to their ability to readily align and form a network with few aggregates and a relatively high fraction of surface bonds. The addition of a polymer brush to an anchored organosilane molecule demonstrates that increased bond tension accelerates the degrafting process despite the increased diffusion resistance.


 Please wait while we load your content...
Please wait while we load your content...