Electron-induced vibrational excitation and dissociative electron attachment in methyl formate
Abstract
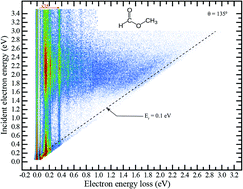
We probe the low-energy electron collisions with methyl formate HCOOCH3, focusing on its resonant states. Experimentally, we (i) use two-dimensional electron energy loss spectroscopy to gain information about the vibrational excitation and (ii) report the absolute dissociative electron attachment cross sections. The electron scattering spectra reveal both the threshold effects due to the long-range electron–molecule interaction and a pronounced π* resonance centered around 2.1 eV. This resonance gives rise to dissociative electron attachment into three different anionic channels, the strongest one being the production of the formate anion. Theoretically, we characterize this resonant state using the complex absorbing potential approach combined with multistate multireference perturbation theory, which predicts its position and width in excellent agreement with the experiment.
- This article is part of the themed collection: 2019 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...
