Revisiting the spectroscopy of xanthine derivatives: theobromine and theophylline†
Abstract
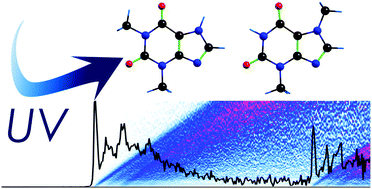
We explore the influence of the relative position of the methyl substituent on the photophysics of theophylline and theobromine, two molecules that are structurally related to the DNA bases. Using a combination of spectroscopic techniques and quantum mechanical calculations, we show that moving the methyl group from N1 in theophylline to N7 in theobromine causes significant differences in their excited state properties, i.e., it produces pyramidalization of N7 in the excited state of the latter. Paradoxically, this modification seems to have little effect on the structural properties of the cation and the ionization process. It is suggested that similar effects may exist in the excited state properties of DNA bases.


 Please wait while we load your content...
Please wait while we load your content...