Intracluster proton transfer in protonated benzonitrile–(H2O)n≤6 nanoclusters: hydrated hydronium core for n ≥ 2†
Abstract
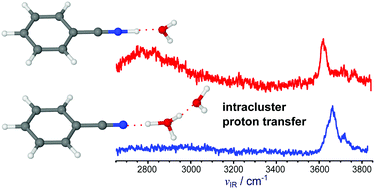
Protonation and hydration of aromatic hydrocarbon molecules and their derivatives play a key role in many biological and chemical processes. The recent detection of benzonitrile (BN, cyanobenzene, C6H5CN) in the interstellar medium suggests the existence of its protonated form (H+BN) in both the gas phase and in or on ice grains. Herein, we analyze the vibrational signatures of size-selected protonated clusters composed of BN and water (W, H2O), H+(BN–Wn=1–6), in the XH stretch range (X = C, N, O) with the aid of dispersion-corrected density functional theory calculations (B3LYP-D3/aug-cc-pVTZ). The size-dependent frequency shifts provide detailed insight about the site of protonation and the structure of the hydration shell. For n = 1, the proton is attached to the N atom of the CN group in BN, and W acts as a proton acceptor in an NH⋯O ionic hydrogen bond (H-bond) of a H+BN–W type structure with cation–dipole configuration. For n ≥ 2, the proton is transferred to the H-bonded hydration network, consistent with thermochemical arguments arising from both the relative proton affinities of BN and Wn and the solvation energies. In these proton-transferred BN–H+Wn structures, the excess proton is more or less localized at a H3O+ hydronium core solvated by neutral W and BN ligands. At least for the considered cluster size (n ≤ 6), the BN impurity molecule is located in the first solvation shell of the H3O+ ion, consistent with the larger electric dipole moment and proton affinity of BN as compared to W. However, the energy gap between these structures and surface isomers with BN solvated further away from the charge decreases with cluster size, suggesting that BN is located at the surface in large BN–H+Wn clusters. While for smaller clusters (n ≤ 4) the hydration network prefers branched structures at T = 0 K, in larger clusters (n ≥ 5) cyclic configurations with four- or five-membered H+Wn rings are most stable because they feature more H-bonds than the branched structures. Comparison with bare H+Wn clusters reveals the substantial effects of the perturbation by the BN impurity on the structure of the hydration network.


 Please wait while we load your content...
Please wait while we load your content...