Water-assisted stability of carbene: cyclic voltammetric investigation of 1-ethyl-3-methylimidazolium ethylsulfate ionic liquid†
Abstract
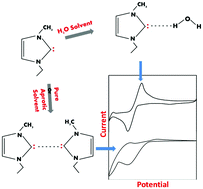
In this work, we report electrochemical studies on imidazolium-based ionic liquids with an objective to explore the possibility of carbene formation in their dilute aqueous solutions. Conventionally, water plays a detrimental role during investigations involving ionic liquids, and this role has been investigated via electrochemical studies in aqueous ionic liquid solutions. There are varying opinions regarding the influence of water on the physicochemical behaviour of ionic liquids that require an in-depth understanding. To eludicate the role of water, we attempted to evaluate the electrochemical performance of ionic liquids in water as a solvent, and the influence of water on ionic liquids was explored through feasibility and stability studies on carbene formed in an aqueous imidazolium-based ionic liquid solution. The electrochemical investigation of an aqueous solution of 1-ethyl-3-methylimidazolium ethylsulfate ([EMIM][EtSO4]) revealed a redox couple. Detailed investigations suggest that reduction of the imidazolium cation occurs at the C2 position, with subsequent formation of carbene. Furthermore, an anodic peak was found to be associated with the oxidation of carbene. The coulometric process associated with the anodic peaks indicated that the two-electron oxidation of carbene occurred. The stability of carbene in water was evaluated through the use of different protic and aprotic solvents. The hydrogen bond-forming ability of carbene with water seems to be responsible for its improved stability in water.


 Please wait while we load your content...
Please wait while we load your content...