Understanding deep dehydrogenation and cracking of n-butane on Ni(111) by a DFT study†
Abstract
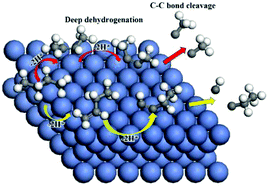
Steam reforming is a main industrial process for hydrogen production. In particular, with the carbon chain increasing to n-butane, a main component in liquefied petroleum gas (LPG) and shale oil gas, chemically different C–C bonds ((C–C)α,β and (C–C)β,β′) will be involved in cleavages. In addition, understanding the role of catalysis in these pathways is critical toward the advancement in technology, yet is largely lacking. As such, we have performed density functional theory (DFT) calculations to study the two possible C–C cleavage pathways of n-butane on Ni(111), i.e., the (C–C)α,β cleavage from the n-butane deep dehydrogenation product of 1-butyne, and the (C–C)β,β′ cleavage from 2-butyne. The results indicate that these two different pathways have distinct dehydrogenations to butyne, and that Ni is suitable for the deep dehydrogenation. The C–C cleavage in both pathways serves as the rate-determining step with a higher energy barrier than that for the preceding C–H bond cleavage. In addition, the 1-butyne pathway was found to be more favorable than that of 2-butyne in thermodynamics and kinetics. Our results provide insights into the alkane dehydrogenation and cracking of long-chain hydrocarbons on Ni-based catalysts.


 Please wait while we load your content...
Please wait while we load your content...