OBCN isomerization and noble gas insertion compounds of identical valence electron number species: stability and bonding†
Abstract
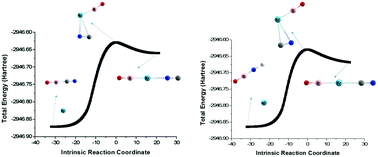
A series of new noble gas (Ng) insertion compounds of the general type XNgX, XNgY and XNgY+ has been theoretically studied using ab initio and DFT methods herein. We first studied the isomerization process of the OBCN compound, and then investigated the bonding properties and stability of the compounds formed by inserting Ng into the single bond of the three low energy isomers by high-level ab initio calculations. The OBNgCN compounds are thermochemically stable with respect to all dissociation channels except for the processes of releasing OBCN/OBNC and free Ng. Furthermore, the two dissociation processes OBNgCN → Ng + OBNC and OBNgNC → Ng + OBCN are kinetically prohibited by the relatively high free energy barrier ranging from 22.7 to 31.7 kcal mol−1 except for the OBKrCN and OBKrNC analogues. And the adaptive natural density partitioning (AdNDP) analysis indicated that chemical bonding in OBNgCN compounds is realized via a delocalized 3-center 2-electron (3c-2e) σ-bond in the B–Ng–C moiety and a totally delocalized 5-center 2-electron (5c-2e) σ-bond in the whole O–B–Ng–C–N. Natural bond orbital (NBO) theory, atoms-in-molecules (AIM) and energy decomposition analysis (EDA) based on the molecular wavefunction revealed that the B–Ng bond and Ng–C bond have some covalent character in OBNgCN. In addition, the calculation and detailed bonding analysis on a large number of neutral and monocationic compounds with identical valence electron numbers to OBNgCN demonstrate that the two bonds directly linked to the Ng atoms have covalent properties in neutral compounds, whereas Ng forms one typical covalent bond and one partial covalent and partial ionic bond with the neighboring atoms in the monocationic compounds.
- This article is part of the themed collection: 2019 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...