Hydrogen polarity of interfacial water regulates heterogeneous ice nucleation
Abstract
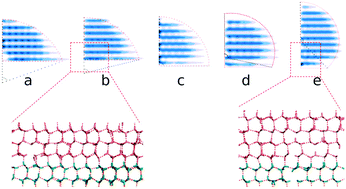
Using all-atomic molecular dynamics (MD) simulations, we show that the structure of interfacial water (IW) induced by substrates characterizes the ability of a substrate to nucleate ice. We probe the shape and structure of ice nuclei and the corresponding supercooling temperatures to measure the ability of IW with various hydrogen polarities for ice nucleation, and find that the hydrogen polarization of IW even with the ice-like oxygen lattice increases the contact angle of the ice nucleus on IW, thus lifting the free energy barrier of heterogeneous ice nucleation. The results show that not only the oxygen lattice order but the hydrogen disorder of IW on substrates are required to effectively facilitate the freezing of top water.


 Please wait while we load your content...
Please wait while we load your content...