Study of odd–even effects in physisorption and chemisorption of Ar, N2, O2 and NO on open shell Ag11–13+ clusters by means of self-consistent van der Waals density functional calculations
Abstract
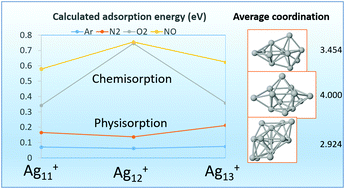
We have studied the adsorption and coadsorption properties of one or more X = Ar, N2, O2, and NO adsorbates on cationic silver clusters Ag11–13+, whose sizes are in the open shell region of metal clusters, aiming to understand the observed odd–even effects in the abundance spectra of Ag11–13+·mX complexes. All calculations were performed self-consistently using a non-local van der Waals correlation functional, covering the different nature of the interactions between the silver substrate and the several adsorbates, which range from dispersion (London) forces for Ar, non covalent π–π interactions for N2, charge-transfer interactions for O2 and NO, and the covalent Ag–Ag bond in the nude silver cluster. Despite the wide interval of adsorption energies, spanning two orders of magnitude, we have been able to explain the following experimental facts. For X = Ar, N2, and O2 reactions with Ag11–13+, it was observed in the mass spectra an abundance peak at n = 12 [M. Schmidt, et al., ChemPhysChem, 2015, 16, 855]. In addition it was observed the competitive adsorption of two or more N2 molecules, and the cooperative effect of adsorbing N2 together with O2 molecules. For X = NO, an abundance peak at n = 12 has been also observed [J. Ma, et al., Phys. Chem. Chem. Phys., 2016, 18, 12819]. We find that the main factors determining these properties are the different core motifs of the cluster geometry (pentagonal bipiramid for Ag11+ and Ag13+, but triangular prism for Ag12+) and, on the other hand, the odd number of valence electrons for Ag12+, leading to a smaller HOMO–LUMO gap than those of its neighbours. Further details about the preferred adsorption sites, dipole moments, and dipole polarizabilities are also discussed.


 Please wait while we load your content...
Please wait while we load your content...