Planarity and out-of-plane vibrational modes of tryptophan and tyrosine in biomolecular modeling†
Abstract
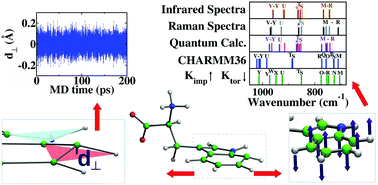
Tryptophan and tyrosine are amino acids that play significant roles in the folding processes of proteins at water–membrane interfaces because of their amphipathic heteroaromatic rings. Employing appropriate heteroaromatic molecular structures is essential for obtaining accurate dynamics and predictive capabilities in molecular simulations of these amino acids. In this study, molecular dynamics simulations that applied the most recent version of the CHARMM36 force field were conducted on aqueous solutions of tryptophan and of tyrosine. Geometric analysis and dynamics quantified how aromatic rings deviated from planar structures and exhibited out-of-plane fluctuations. Radial distribution functions showed possible biological significance because the extent of ring planarity slightly affected local water concentrations near aromatic rings. Instantaneous all-atom normal mode analysis (NMA) and Fourier transformation of time autocorrelation functions of out-of-plane displacements were applied to study out-of-plane vibrations of atoms in these rings. The NMA started with minimum energy configurations and then averaged over fluctuations in aqueous solution. The frequencies and frequency patterns that were obtained for tryptophan and tyrosine with CHARMM36 differed from literature reports of Raman spectra, infrared spectra, and frequencies calculated using quantum mechanics, with some out-of-plane modes found at higher frequencies. Effects of imposing improper torsion potentials and changing torsion angle force constants were investigated for all atoms in the rings of tryptophan and tyrosine. Results show that these coarse force field variations only affect planarity and out-of-plane vibrations of atoms within the rings, and not other vibrations. Although increasing improper torsion force constants reduced deviations from aromatic ring planarity significantly, it increased out-of-plane mode frequencies. Reducing torsion angle force constants (with and without improper torsions) shifted modes to lower frequencies. A combination of decreasing most torsion angle force constants for ring atoms in both amino acids and including improper torsion forces attained frequencies and frequency patterns for out-of-plane normal modes that were more similar to the literature spectra. These force field variations decreased the extents of out-of-plane vibrations within the heteroaromatic rings of tryptophan, especially around the nitrogen atom in the ring, but not within the heteroaromatic ring of tyrosine. Conclusions were unaffected by the peptide endgroup, water, or simulation ensemble.


 Please wait while we load your content...
Please wait while we load your content...