Spin–orbit coupling in magnetoelectric Ba3(Zn1−xCox)2Fe24O41 hexaferrites†
Abstract
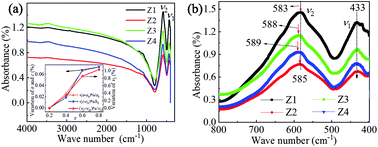
The Z-type hexaferrites Ba3(Zn1−xCox)2Fe24O41 (x = 0.2, 0.4, 0.6, 0.8, defined as Z1–Z4) were synthesized by a sol–gel method. With increasing cobalt concentration, the origin of magnetoelectric (ME) coupling and the effects of crystal parameters, occupation of ions, and magnetocrystalline anisotropy (MCA) on ME current were studied systematically. The mechanism of magnetic phase transition, revealing the evolution of the magnetic order in the temperature range of 10–400 K, was discussed in detail. Our results suggest that the ferroelectricity of Z1–Z4 originates from both inverse Dzyaloshinskii Moriya (DM) interaction and p–d hybridization mechanism. In particular the ME coupling property is only dominated by p–d hybridization with spin–orbit coupling. This study provides an effective way to improve the ME coupling property of hexaferrites, which have potential applications in the design of new electronic devices.


 Please wait while we load your content...
Please wait while we load your content...