Falloff curves and mechanism of thermal decomposition of CF3I in shock waves†
Abstract
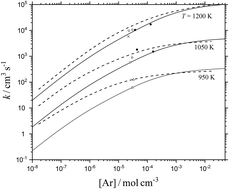
The falloff curves of the unimolecular dissociation CF3I (+Ar) → CF3 + I (+Ar) are modelled by combining quantum-chemical characterizations of the potential energy surface for the reaction, standard unimolecular rate theory, and experimental information on the average energy transferred per collision between excited CF3I and Ar. The (essentially) parameter-free theoretical modelling gives results in satisfactory agreement with data deduced from earlier shock wave experiments employing a variety of reactant concentrations (between a few ppm and a few percent in the bath gas Ar). New experiments recording absorption–time signals of CF3I, I2, CF2 and (possibly) IF at 450–500 and 200–300 nm are reported. By analysing the decomposition mechanism, besides the unimolecular dissociation of CF3I, these provide insight into the influence of secondary reactions on the experimental observations.
- This article is part of the themed collection: 2019 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...