Low energy optical excitations as an indicator of structural changes initiated at the termini of amyloid proteins†
Abstract
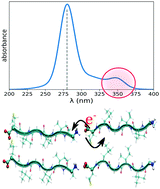
There is a growing body of experimental work showing that protein aggregates associated with amyloid fibrils feature intrinsic fluorescence. In order to understand the microscopic origin of this behavior observed in non-aromatic aggregates of peptides and proteins, we conducted a combined experimental and computational study on the optical properties of amyloid-derived oligopeptides in the near-UV region. We have focused on a few model systems having charged termini (zwitterionic) or acetylated termini. For the zwitterionic system, we were able to simulate the longer tail absorption in the near UV (250–350 nm), supporting the experimental results in terms of excitation spectra. We analyzed the optical excitations responsible for the low-energy absorption and found a large role played by charge-transfer states around the termini. These charge-transfer excitations are very sensitive to the conformation of the peptide and in realistic fibrils may involve inter and intra chain charge reorganization.
- This article is part of the themed collection: 2019 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...