Ab initio dynamics of hydrogen abstraction from N2H4 by OH radicals: an RRKM-based master equation study†
Abstract
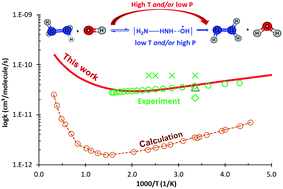
The detailed reaction mechanism of the N2H4 + OH reaction is comprehensively reported for a wide range of conditions (i.e., T = 200–3000 K & P = 1–7600 Torr) using the CCSD(T)/CBS//M06-2X/6-311++G(3df,2p) level and the master equation/Rice–Ramsperger–Kassel–Marcus (ME/RRKM) rate model, which includes corrections of the hindered internal rotor (HIR) and tunneling treatments. Our calculated rate constants are found in excellent agreement with the latest experimental data (G. L. Vaghjiani, Int. J. Chem. Kinet., 2001, 33, 354–362), which helps to resolve the discrepancy between the previous experimental and theoretical studies. The reaction mechanism is revealed as: (i) the H-abstraction channel is more thermodynamically favorable than the OH-substitution mechanism; (ii) non-Arrhenius behaviors and slightly positive pressure-dependence at low temperature (T ≤ 500 K) of the rate coefficients are observed and (iii) the HIR treatment plays a substantial role in obtaining the reliable rate constants. Moreover, the performance of several molecular electronic structure methods (i.e., M06-2X, B3LYP, BH&HLYP and MP2) on the rate coefficient calculations is also discussed thoroughly in this work.


 Please wait while we load your content...
Please wait while we load your content...