A space-confined strategy toward large-area two-dimensional crystals of ionic liquid†
Abstract
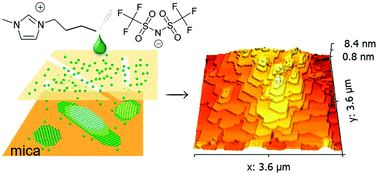
Understanding and manipulating nano-confined ionic liquids (ILs) has tremendous implications in nanotechnology and chemical engineering. Here, a peculiar growth phenomenon of a nano-confined [Bmim][NTFI] ionic liquid is revealed by utilizing two-dimensional channels in mica. The intercalated ILs underwent liquid–solid transition and self-assembled into a self-similar two-dimensional crystal in an epitaxial relation with the confining material. The terraced IL crystals, ranging from monolayer to bilayer to several dozen layers, are characterized by unexpectedly large areas extending to μm-scale and enhanced thermal stability with a melting temperature 73 K higher than that of the corresponding bulk IL. The notable asymmetric feature of the layered crystals hints at anisotropic growth under confinement, which produces a well-defined hexagonal geometric shape. Finally, a molecular scale growth mechanism of ordered ILs is qualitatively interpreted by a birth-and-spread model. Our findings have enabled new research on nanoconfined ILs and opened up an avenue to tailoring the structure of ILs for their applications under confinement.


 Please wait while we load your content...
Please wait while we load your content...