Transition of interfacial capacitors in electrowetting on a graphite surface by ion intercalation†
Abstract
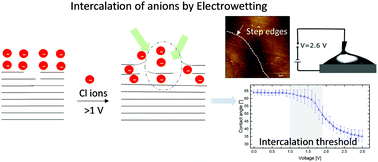
The low voltage electrowetting response of a LiCl aqueous solution on a freshly cleaved surface of highly oriented pyrolytic graphite (HOPG) is presented. For applied voltages below 1 V, the energy stored in the electrical double layer (EDL) is insufficient to drive the spreading of the drop due to the pinning of the three phase contact line at the step edges. Electrochemical impedance spectroscopy shows a dramatic increase in capacitance above 1 V, which provides a sufficient electrowetting force for depinning the contact line, resulting in a subsequent decrease of the contact angle. The transition of the interfacial capacitance from the EDL to the many-fold high capacitance of the pseudocapacitor drives the electrowetting transition on the HOPG surface. The observed changes in the capacitances above 1 V are correlated with the cyclic voltammetry and atomic force microscopy results, which show that the Cl− ions intercalate into the graphite galleries upon acquiring sufficient energy to overcome the van der Waals attraction between the graphene layers through the side of the step edge of the basal planes. To the best of our knowledge, this is the first study on the voltage dependent intercalation mediated transition of interfacial capacitance driving the spreading of an aqueous electrolyte drop on the HOPG surface, which provides a fundamental understanding of the mechanism and opens up potential applications in microfluidics and charge storage technologies.


 Please wait while we load your content...
Please wait while we load your content...