Understanding propyl cyanide and its isomers formation: ab initio study of the spectroscopy and reaction mechanisms†
Abstract
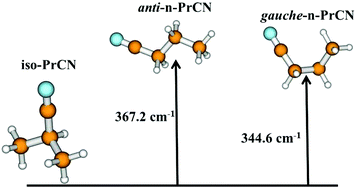
Recent detection of propyl cyanide (C3H7CN) with both linear and branched structures has stimulated many experimental and theoretical studies. In this theoretical work, we present the spectroscopic properties of the far infrared spectra of these species and we investigate their different paths of formation in the gas phase. Our spectroscopic study concerns the far infrared spectra of iso, anti and gauche propyl cyanide isomers. The equilibrium structures and the potential energy surfaces are calculated using explicitly correlated cluster ab initio methods (CCSD(T)-F12) and a variational procedure designed for non-rigid species and large amplitude motions. Accurate rotational constants, centrifugal distortion constants, potential energy barriers and surfaces are provided. The rovibrational parameters in the ground vibrational states compare very well with experimental data. The low energy vibrational levels correspond to torsional modes. Far infrared energies are calculated up to 500 cm−1 using the variational approach and the vibrational second order theory (VPT2), and a good agreement with previous experimental values is found. We have also investigated the gas phase formation of the different C3H7CN isomers. After several trials of reacting gaseous species, we considered that a possible formation route of the C3H7CN isomers can be from the bimolecular reaction of HCN with propene. At the UMP2(full)/aug-cc-pVTZ level of theory, this reaction involves two steps for each isomer; the first one corresponds to the association of the two radicals while the second one corresponds to H transfer. From highly correlated ab initio calculations by means of CCSD(T)/aug-cc-pVTZ//UMP2(full)/aug-cc-pVTZ, the geometries, energetics and minimum energy paths of the reactions are obtained. Also, the first step's transition state disappears, as the diradical minimum is much less stabilized with UCCSD(T) than by UMP2(full). We employ the zero curvature tunneling and canonical variational (CVT/ZCT) semiclassical method to predict rate constants for propyl cyanide isomers formation in the gas phase. However, due to the presence of a significant barrier, this reaction leads to very small rate constants. Very recently, we probed reaction mechanisms involving radical addition and we found that these reactions are barrierless and highly exothermic leading to expected fast reactive processes for the formation of propyl cyanide products. The kinetics of such diradical reactions is under study.
- This article is part of the themed collection: 2019 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...