Investigation of glycerol hydrogen-bonding networks in choline chloride/glycerol eutectic-forming liquids using neutron diffraction†
Abstract
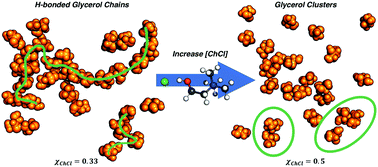
The structure of choline chloride/glycerol (ChCl : Gly) mixtures at two mole fractions (the eutectic χChCl = 0.33 (1 : 2), and a higher χChCl = 0.50 (1 : 1) composition) in the liquid state at 333 K and 1 atm. has been investigated using neutron diffraction coupled with hydrogen/deuterium isotopic substitution. Modelling using the empirical potential structure refinement (EPSR) technique, constrained to the experimental neutron diffraction data, produced structural models at both compositions consistent with the experimental data with an extensive, persistent homo-molecular glycerol hydrogen bonding network at χChCl = 0.33 similar to that present in pure glycerol and suggests that persistence of the latent glycerol hydrogen bonding network is key to formation of the ChCl : Gly deep eutectic solvent. In the choline chloride-rich χChCl = 0.50 composition, significant domain segregation is observed with a dramatic reduction in the extent of the homo-molecular glycerol hydrogen bond network which is replaced by a more homogeneous system-wide hydrogen bonded network incorporating glycerol, Cl−, and choline cations.
- This article is part of the themed collection: 2019 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...