Elucidating the formation of Al–NBO bonds, Al–O–Al linkages and clusters in alkaline-earth aluminosilicate glasses based on molecular dynamics simulations†
Abstract
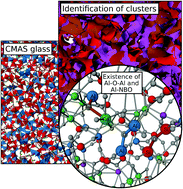
Exploring the reasons for the initiation of Al–O–Al bond formation in alkali-earth alumino silicate glasses is a key topic in the glass-science community. Evidence for the formation of Al–O–Al and Al–NBO bonds in the glass composition 38.7CaO–9.7MgO–12.9Al2O3–38.7SiO2 (CMAS, mol%) has been provided based on Molecular Dynamics (MD) simulations. Analyses in the short-range order confirm that silicon and the majority of aluminium cations form regular tetrahedra. Well-separated homonuclear (Si–O–Si) and heteronuclear (Si–O–Al) cluster regions have been identified. In addition, a channel region (C-Region), separated from the network region, enriched with both NBO and non-framework modifier cations, has also been identified. These findings are in support of the previously proposed extended modified random network (EMRN) model for aluminosilicate glasses. A detailed analysis of the structural distributions revealed that a majority of Al, 51.6%, is found in Si–O–Al links. Although the formation of Al–O–Al and Al–NBO bonds is energetically less favourable, a significant amount of Al is found in Al–O–Al links (33.5%), violating Lowenstein's rule, and the remainder is bonded with non-bridging oxygen (NBO) in the form of Al–NBO (Al–O–(Ca, Mg)). The conditions necessary for the formation of less favourable bonds are attributed to the presence of a high amount of modifier cations in current CMAS glass and their preferable coordination.


 Please wait while we load your content...
Please wait while we load your content...