Electronic engineering of a tetrathiafulvalene charge-transfer salt via reduced symmetry induced by combined substituents†‡
Abstract
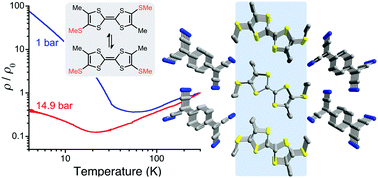
A 1 : 1 metallic charge-transfer salt is obtained by cosublimation of (Z,E)-(SMe)2Me2TTF and TCNQ. X-ray diffraction studies confirm the formation of segregated stacks comprising donor and acceptor molecules in [(E)-(SMe)2Me2TTF](TCNQ). The crystal packing features lateral S⋯S interactions between TTF stacks, which is in sharp contrast to that in (TTF)(TCNQ). Structural analysis and theoretical studies afford a partial charge-transfer (ρ ≈ 0.52), leading to a system with the electronic structure close to quarter-filled. Resistivity measurements reveal that this material behaves as a metal down to 56 K and 22 K at 1 bar and 14.9 kbar, respectively. The thermopower is negative in the metallic regime, indicating the dominant role of the acceptor stacks for the observed conducting behavior. Analysis of single-crystal EPR spectra shows the remaining spin susceptibility at 4.3 K, suggesting the importance of the Hubbard U correction. These results highlight the judicious engineering of electronic and geometrical effects on the TTF core; the combined use of methyl and thiomethyl groups has decreased the TCNQ bandwidth while maintaining the segregated stacks, converting the metal to insulator (M–I) transition to more 4kF like. In addition, the enhanced S⋯S contacts between the TTF stacks lead to more rapidly decreasing M–I transition temperature under various pressures.


 Please wait while we load your content...
Please wait while we load your content...