Molecular dynamics investigation of reduced ethylene carbonate aggregation at the onset of solid electrolyte interphase formation
Abstract
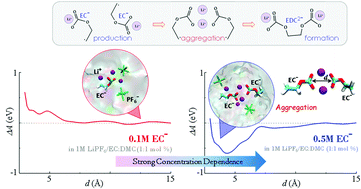
Formation of the solid electrolyte interphase (SEI) at the anode surface during the initial charge cycles is critical to lithium ion battery operability. Reduction of electrolyte components must ultimately result in the formation of this ionically conducting and electronically insulating layer to compensate for the limited electrochemical stability window of conventional liquid electrolytes. One important reaction in SEI formation is the bimolecular combination of radical anions to form more stable products. Molecular dynamics simulations illustrate the nature and dynamics of the intermolecular interactions between the reactive intermediates produced from one-electron reduction in ethylene carbonate-based electrolytes. A clear concentration dependence is shown for this interaction, and its ramifications for SEI formation are discussed.


 Please wait while we load your content...
Please wait while we load your content...