Photoinduced electron transfer and unusual environmental effects in fullerene–Zn-porphyrin–BODIPY triads†
Abstract
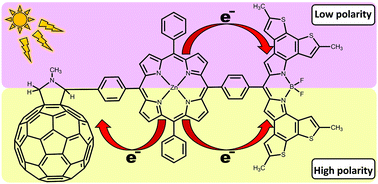
Molecular arrays containing donor–acceptor sites and antenna molecules are promising candidates for organic photovoltaic devices. Photoinduced electron transfer (PET) in multi-chromophore systems is controlled by a subtle interplay of donor and acceptor properties and solvent effects. In the present study, we explore how PET of fullerene [C60]–Zn-porphyrin–BODIPY triads can be modulated by passing from non-polar to polar media. To this end we perform a computational study of this complex using the DFT/TDDFT method. We find that the stabilization energy of charge transfer states by a polar medium depends significantly on whether the BODIPY moiety acts as an electron donor or an electron acceptor. To understand this effect of the environment, a detailed analysis of the initial and final states of the ET reactions is performed. We show that additional deactivation channels of the porphyrin excited state may come into play as solvent polarity increases.


 Please wait while we load your content...
Please wait while we load your content...