In silico investigation of the interaction between the voltage-gated potassium channel Kv4.3 and its auxiliary protein KChIP1†
Abstract
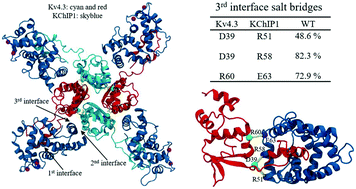
The voltage-gated potassium channel Kv4.3 plays a vital role in shaping the timing, frequency, and backpropagation of electrical signals in the brain and heart by generating fast transient currents at subthreshold membrane potentials in repetitive firing neurons. To achieve its physiological function, Kv4.3 is assisted by auxiliary β-subunits that become integral parts of the native A-type potassium channels, among which there are the Kv channel-interacting proteins (KChIPs). KChIPs are a family of cytosolic proteins that, when coexpressed with Kv4, lead to higher current density, modulation of channel inactivation and faster recovery from inactivation, while the loss of KChIP function may lead to severe pathological states. Recently, the structural basis of the KChIP1–Kv4.3 interaction was reported by using two similar X-ray crystallographic structures, which supported a crucial role for KChIP1 in enhancing the stability of the Kv4.3 tetrameric assembly, thus helping the trafficking of the channel to the plasma membrane. Here, we investigate through fully atomistic simulations the structure and stability of the human Kv4.3 tetramerization (T1) domain in complex with KChIP1 upon specific mutations located in the first and second interfaces of the complex, as compared to the wild-type (WT). Our results nicely complement the available structural and biophysical information collected so far on these complex variants. In particular, the degree of structural deviations and energetic instability, from small to substantial, observed in these variants with respect to the WT model seems to parallel well the level of channel dysfunction known from electrophysiology data. Our simulations provide an octameric structure of the WT KChIP1-Kv4.3 assembly very similar to the known crystal structures, and, at the same time, highlight the importance of a previously overlooked site of interaction between KChIP1 and the Kv4.3 T1 domain.


 Please wait while we load your content...
Please wait while we load your content...