On the applicability of functional-group symmetry-adapted perturbation theory and other partitioning models for chiral recognition – the case of popular drug molecules interacting with chiral phases†
Abstract
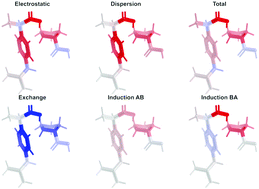
The applicability of symmetry-adapted perturbation theory (SAPT) and functional-group SAPT (F-SAPT) to study chiral recognition is investigated on an example of three popular chiral drug molecules: ibuprofen, norepinephrine, and baclofen, interacting with phenethylamine or proline – two molecules that are often used as chiral phases in chromatography. The comparison of the F-SAPT with the interacting quantum atoms (IQA) approach is also provided. Accurate estimation of energetic differences of the non-covalent intermolecular complexes composed of two chiral molecules is a necessary prerequisite for the possibility of a prediction of the chiral recognition. The SAPT method with interacting molecules described on the density functional theory level provides accurate total interaction energies, while the F-SAPT approach is the most useful in determining which functional groups are responsible for strengthening or weakening of the interaction between chiral molecules. The largest difference in the interaction energies has been calculated for the baclofen–phenethylamine and norepinephrine–phenethylamine pairs, while the smallest for the ibuprofen–proline and baclofen–proline ones. In most cases, the intermolecular interaction is found to be composed of a strong directional hydrogen bond, which was stabilized by two or more weaker non-covalent interactions between groups (in accordance with the phenomological three-point rule), but in several cases more subtle factors are responsible for larger stability of one diastereoisomer, like the stabilization of the conformation involving two noninteracting functional groups attached to a chiral atom through intramolecular attraction. Additionally, the simulated IR spectra were analyzed for all pairs of diastereoisomeric complexes and the red- and blue-shifts of characteristic bond vibrations were discussed in the context of inter-group interactions.


 Please wait while we load your content...
Please wait while we load your content...