Observation of the dipole- and quadrupole-bound anions of 1,4-dicyanocyclohexane
Abstract
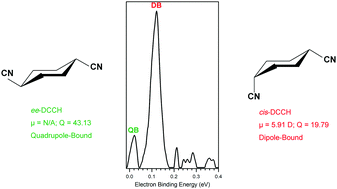
Quadrupole-bound anions are negative ions in which their excess electrons are loosely bound by long-range electron-quadrupole attractions. Experimental evidence for quadrupole-bound anions has been scarce; until now, only trans-succinonitrile had been experimentally confirmed to form a quadrupole-bound anion. In this study, we present experimental evidence for a new quadrupole-bound anion. Our combined Rydberg electron transfer/anion photoelectron spectroscopy study demonstrates that the ee conformer of 1,4-dicyanocyclohexane (DCCH) supports a quadrupole-bound anion state, and that the cis-DCCH conformer forms a dipole-bound anion state. The electron binding energies of the quadrupole- and dipole-bound anions are measured as 18 and 115 meV, respectively, both of which are in excellent agreement with theoretical calculations by Sommerfeld.


 Please wait while we load your content...
Please wait while we load your content...