Evolutionary search for (M©B16)Q (M = Sc–Ni; Q = 0/−1) clusters: bowl/boat vs. tubular shape†
Abstract
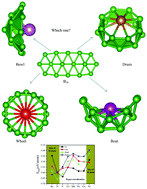
Herein, using universal structure predictor: evolutionary xtallography (USPEX) method, followed by density functional theory (DFT) calculations, we performed global searches for the most stable structures of (M©B16)Q (M = Sc, Ti, V, Cr, Mn, Fe, Co, Ni; Q = 0, −1) clusters. It was found that the obtained ground-state structures of (M©B16)Q clusters exhibited a distinct structural evolution as M changed from V to Ni: from bowl-shaped, to boat-shaped, to an M-centered tubular structure named wheel-shaped, to drum-shaped (the metal atom was adsorbed on top of the cross section of the B16 species). Our analysis shows that hyper-coordination and the size of the metal atom are two competing factors determining the relative stability and topological properties of the (M©B16)0/−1 clusters, resulting in unprecedented structures for Sc, Ti, and Ni-doped clusters. The calculated binding energies for these new configurations are even larger than those of the previously synthesized B16−1, (Mn©B16)−1, and (Co©B16)−1 clusters, indicating their very good stability and possible experimental synthesis. A net charge transfer from the metal atom to the boron moiety occurs for all clusters, indicating that electrostatic interactions play an important role in the stability of these materials. Finally, the Sc©M16 and Ti©B16 clusters exhibit not only excellent thermal stability but also large first hyper-polarizability. Hence, they are expected to be potential innovative candidates for excellent electro-optical materials.


 Please wait while we load your content...
Please wait while we load your content...