Evidencing the relationship between isomer spectra and melting: the 20- and 55-atom silver and gold cluster cases†
Abstract
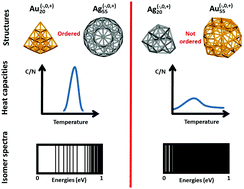
The present work highlights the links between melting properties and structural excitation spectra of small gold and silver clusters. The heat capacity curves are computed for Ag20, Au20, Ag55, Au55 and their ions, using a parallel-tempering molecular dynamics scheme to explore the density functional based tight binding (DFTB) potential energy surfaces and the multiple histogram method. It is found that clusters having very symmetric lowest energy structures (Au20, Ag55 and their ions) present sharp or relatively sharp solid-to-liquid transitions and large melting temperatures, important structural excitation energies and a discrete isomer spectrum. Opposite trends are observed for less ordered clusters (Ag20, Au55 and their ions). Regarding the structural evolution with temperature, very symmetric clusters exhibit minor evolution up to the starting melting temperature. The present study also highlights that, in contrast with the case of Au20, a single electron excess or deficiency is not determinant regarding the melting characteristics, even quantitatively, for clusters containing 55 atoms, for gold as for silver.


 Please wait while we load your content...
Please wait while we load your content...