The solvation effect on the rattling behaviour of the hydrated excess proton in water†
Abstract
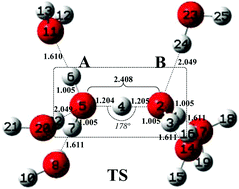
The solvation effect on the kinetic rattling behaviour of the hydrated excess proton H+(aq) in water is theoretically modeled by using density functional theory (DFT) and the quantum chemical cluster model (CM). To test the solvation effects on the proton morphology and rattling kinetics, different solvation models for the proton are constructed based on the gas phase (GP) Zundel cation, which include the gas phase-polarizable continuum model (GP-PCM), the gas phase-supermolecule model (GP-SM), and the gas phase-supermolecule-polarizable continuum model (GP-SM-PCM). These solvation models consider either one or both of the short- and long-range solute–solvent interactions. Meanwhile, 1 to 6 explicit solvent water molecules (Nm′ = 1–6) are added around the GP Zundel cation to test different explicit solvation environments. The calculation results show that the solvation environment has an important influence on the morphology and rattling kinetics of H+(aq). The proton rattling pathways are obtained only under the condition that both symmetrical explicit solvation environments and implicit bulk solvents are present. The zero-point contribution reduces the reaction energy barrier and enables the rattling to occur spontaneously at room temperature. The theoretical modeling results provide new insights into the microscopic kinetic behaviour of proton rattling in water at the molecular level, which are helpful in studying the proton transfer mechanism in aqueous systems.


 Please wait while we load your content...
Please wait while we load your content...