Why hydroxy-proline improves the catalytic power of the peptidoglycan N-deacetylase enzyme: insight from theory†
Abstract
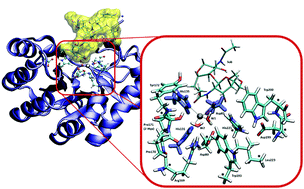
Nature exploits different strategies for enhancing the catalytic activity of enzymes, often resorting to producing beneficial mutations. The case of post-translational proline hydroxylation mutation in the active site of polysaccharide deacetylase (PDA) Bc1960 from Bacillus cereus is an interesting example of how small chemical modifications can cause significant improvements in enzymatic activity. In the present study the deacetylation mechanism promoted by both OH-proline (2Hyp) and standard proline (Pro) containing PDA is investigated using density functional theory. Although the mechanism presented for the two examined enzymes is in agreement with protease catalysis in metalloenzymes, the analysis along the potential energy surface (PES) reveals that the intermediate and product benefit energetically from the presence of the hydroxyl group on the proline. Our calculations provide evidence that for PDA-2Hyp, the hydrogen bond network established by the –OH group on the Cα of the proline with its closest neighbors stabilizes the transition states and, consequently, the reaction takes advantage of this. These results further contribute towards explaining the different catalytic activity experimentally observed for the polysaccharide deacetylase enzymes.
- This article is part of the themed collection: 2019 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...