Gauging stability and reactivity of carbonyl O-oxide Criegee intermediates†
Abstract
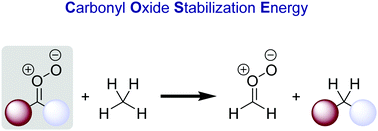
In this study, we evaluated the effect of substitution on the stability and reactivity of carbonyl O-oxide Criegee intermediates (CIs). In this regard, we computed a set of more than 50 carbonyl oxides at the CBS-QB3 level of theory and assessed their stability by means of an isodesmic reaction equation defining a carbonyl oxide stabilization energy (COSE). Almost all substituents are stabilizing and amino groups in particular leading to COSE values of almost 60 kcal mol−1. As opposed to π-donors, substituents with a strong σ-electron pull destabilize the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O–O group. Furthermore, we studied how the intrinsic stabilization of the Criegee intermediate is reflected in its C
O–O group. Furthermore, we studied how the intrinsic stabilization of the Criegee intermediate is reflected in its C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and O–O bond lengths as well as the partial charges on the individual atoms of the carbonyl oxide moiety. As a potential measure for reactivity, we determined the adiabatic singlet–triplet energy gap of all carbonyl oxides. Amino substituted CIs exhibit high-lying triplet states and have relatively large barriers towards addition of water or the OH radical. However, the ΔES–T cannot serve as a rigorous measure for carbonyl oxide reactivity.
O and O–O bond lengths as well as the partial charges on the individual atoms of the carbonyl oxide moiety. As a potential measure for reactivity, we determined the adiabatic singlet–triplet energy gap of all carbonyl oxides. Amino substituted CIs exhibit high-lying triplet states and have relatively large barriers towards addition of water or the OH radical. However, the ΔES–T cannot serve as a rigorous measure for carbonyl oxide reactivity.


 Please wait while we load your content...
Please wait while we load your content...