Molecular anchoring to oxide surfaces in ultrahigh vacuum and in aqueous electrolytes: phosphonic acids on atomically-defined cobalt oxide†
Abstract
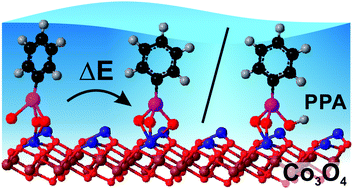
In this work, we investigated the interaction of phenylphosphonic acid (PPA, C6H5PO3H2) with atomically-defined Co3O4(111) thin films, grown on Ir(100), under ultrahigh vacuum (UHV) conditions and in the electrochemical environment. In the first step, we employed infrared reflection absorption spectroscopy (IRAS) and followed the formation of a saturated monolayer (380 K) in UHV. We observed that the binding motif changes from a chelating tridentate in the sub-monolayer regime to a chelating bidentate at full monolayer coverages. In the electrochemical environment, we analyzed the interaction of PPA with the same Co3O4(111) surface by electrochemical infrared reflection absorption spectroscopy (EC-IRRAS) (0.3 VRHE–1.3 VRHE). When adsorbed at pH 10 from an ammonia buffered aqueous solution, PPA binds to the surface in form of a fully deprotonated chelating bidentate. With increasing electrode potential, we observed two fully reversible processes. At low buffer concentration, protons are released upon oxidation of surface Co2+ ions and lead to protonation of the anchored phosphonates. At high buffer concentration, most of the protons released are accepted by NH3. Simultaneously, the surface phosphonate changes its adsorption motif from bidentate to tridentate while adopting a more upright geometry.
- This article is part of the themed collection: 2019 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...