Why is the cubic structure preferred in newly formed ice?†
Abstract
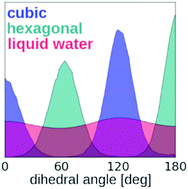
Molecular dynamics was employed to explain the preference for the cubic structure in newly formed crystals of ice. The results showed that in supercooled liquid water the molecules connected by hydrogen bonds are more likely to adopt relative orientations similar to the ones characteristic for cubic ice. The observed preference for certain relative orientations of molecules in the hydrogen-bonded pairs results in the higher probability of the formation of ice with the cubic structure. On that basis, it was concluded that the main reason for the increased probability of the formation of cubic ice in solidifying water is the distinctive structure of liquid water.
- This article is part of the themed collection: 2019 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...