What a difference a methyl group makes – probing choline–urea molecular interactions through urea structure modification†
Abstract
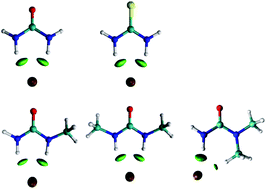
There is a lack of fundamental knowledge on deep eutectic solvents, even for the most extensively studied mixtures, such as the mixture of cholinium chloride and urea, which prevents a judicious choice of components to prepare new solvents. The objective of this work is to study and understand the fundamental interactions between cholinium chloride and urea that lead to the experimentally observed melting temperature depression. To do so, the structure of urea was strategically and progressively modified, in order to block certain interaction centres, and the solid–liquid equilibrium data of each new binary system was experimentally measured. Using this approach, it was concluded that the most important interaction between cholinium chloride and urea occurs through hydrogen bonding between the chloride anion and the amine groups. Any blockage of these groups severely hampers the melting point depression effect. Raman spectroscopy and DFT calculations were utilized to study in more detail this hydrogen bonding and its nuances.


 Please wait while we load your content...
Please wait while we load your content...