Characterization of the simplest hydroperoxide ester, hydroperoxymethyl formate, a precursor of atmospheric aerosols†
Abstract
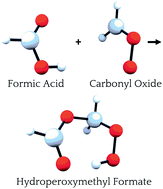
Atmospheric aerosols are large clusters of molecules and particulate matter that profoundly affect the Earth's radiation budget and climate. Gas-phase oxidation of volatile organic compounds is thought to play a key role in nucleation and aerosol growth, but remains poorly understood. One reaction proposed to trigger formation of condensable, low volatility organic compounds is that between Criegee intermediates and carboxylic acids to yield hydroperoxide esters. Here we isolate in high yield the simplest hydroperoxide ester, hydroperoxymethyl formate (HOOCH2OCHO), as a secondary product in the ozonolysis of ethylene, and establish by rotational spectroscopy that this ester adopts a nearly-rigid cyclic structure owing to a strong hydrogen bond between the peroxy hydrogen and carbonyl oxygen. Subsequent detection of this ester in the ozonolysis of propylene and isoprene suggests that terminal alkenes readily undergo specific types of second-order oxidation reactions that have been implicated in the formation of atmospheric aerosols.


 Please wait while we load your content...
Please wait while we load your content...