Modeling of the phase transition inside graphene nanobubbles filled with ethane
Abstract
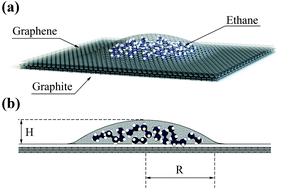
Graphene nanobubbles consist of a substance that is trapped between graphene sheets and atomically flat substrates. This substance is an example of confinement in which both the bulk and surface interactions and the tension of the graphene determine the mechanical and thermodynamic properties of the system. The van der Waals pressure build up due to the graphene–substrate attraction and surface influence facilitates the advanced condensation of trapped substances. Different phases of the trapped substance are assumed to be found inside the graphene nanobubbles depending on their radii. Smaller radii are attributed to the crystal and liquid phases, and larger radii correspond to the gas phase. In this study, graphene nanobubbles filled with ethane on a graphite substrate are investigated. The choice of trapped substance is inspired by typical experiments in which graphene nanobubbles are obtained with a mixture of hydrocarbons inside. We apply a multiscale model based on both molecular dynamics simulations and a continuum 1D model to obtain the shape of the bubble, stress distribution and phase state of the trapped substance. Calculations are performed for a set of temperatures below and above the critical temperature of ethane. A liquid–gas phase transition below the critical temperature leads to a ‘forbidden range’ of radii, in which no stable bubbles exist.
- This article is part of the themed collection: 2019 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...