Resolving the chemical identity of H2SO4 derived anions on Pt(111) electrodes: they're sulfate†
Abstract
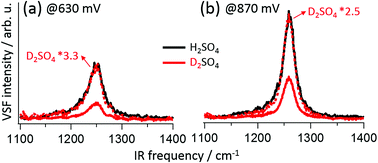
Understanding how electrolyte composition controls electrocatalytic reactions requires molecular-level insight into electrode/electrolyte interaction. Perhaps the most basic aspect of this interaction, the speciation of the interfacial ion, is often controversial for even relatively simple systems. For example, for Pt(111) in 0.5 M H2SO4 it has long been debated whether the adsorbed anion is SO42−, HSO4− or an H3O+⋯SO42− ion pair. Here we apply interface-specific vibrational sum frequency (VSF) spectroscopy and theory to this problem and perform an isotope exchange study: we collect VSF spectra of Pt(111) in H2SO4(H2O) and D2SO4(D2O) as a function of bias and show that at all potentials they are identical. This is the most direct spectroscopic evidence to date that SO42− is the dominant adsorbate, despite the fact that at 0.5 M H2SO4 bulk solution is dominated by HSO4−. This approach is based on the unique selection rule of the VSF spectroscopy and thus offers a new way of accessing general electrode/electrolyte interaction in electrocatalysis.


 Please wait while we load your content...
Please wait while we load your content...