Intrinsically low thermal conductivity of bismuth oxychalcogenides originating from interlayer coupling
Abstract
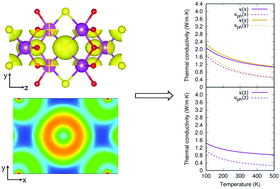
The anharmonicity of phonons in a solid is ultimately rooted in the chemical bonding. However, the direct connection between phonon anharmoncity and chemical bonding is difficult to make experimentally or theoretically, mainly due to their complicated lattice structures. Here, with the help of first-principles calculations, we show that the intrinsically low lattice thermal conductivity (κ) of Bi2O2X (X = S, Se, Te) shows a strong connection to the electrostatic inter-layer coupling. We explain our results by the strong anharmonic chemical bonding between Bi and chalcogen atoms. Additionally, due to the strong anharmonicity, a large portion of phonon modes has a mean free path shorter than the average atomic distance. We employ a recently proposed two-channel model to take into account their contribution to κ.


 Please wait while we load your content...
Please wait while we load your content...