Formation of large clusters of CO2 around anions: DFT study reveals cooperative CO2 adsorption†
Abstract
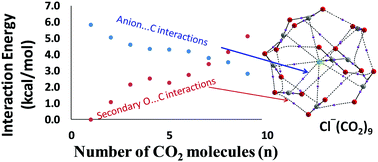
The structure and energetics of the interaction of CO2 molecules with anions F−, Cl−, Br−, CN−, NC−, OH−, ClO−, NH2−, and NO2−, have been studied at the M06L/6-311++G** level of density functional theory. The maximum number of CO2 molecules (nmax) adsorbed by the anions to saturate the first shell of coordination varies from 8 to 12 in different complexes. The anion⋯CO2 distance (dint) in F−(CO2), NC−(CO2), ClO−(CO2), HO−(CO2) and H2N−(CO2) is 1.533, 1.527, 1.468, 1.456, and 1.470 Å, respectively, which indicates covalent bond formation between carbon and the anion, which is confirmed from the interaction energy (Eint) values of these complexes 29.0, 14.7, 23.2, 41.7, and 48.1 kcal mol−1, respectively. The Cl−, Br−, CN− and NO2− interact always non-covalently with the carbon center of CO2 with dint in the range of 2.5–2.9 Å. With the adsorption of each CO2, an average increment of 5.9–6.7 kcal mol−1 is observed in the Eint value of the complexes. The Eint per CO2 (Eint/CO2) is nearly a constant for all the non-covalent complexes, even up to nmax number of CO2 adsorbed. Though the primary anion⋯CO2 interaction gets weaker with the increasing size of the CO2 cluster, a steady increase in the secondary O⋯C interaction between adsorbed CO2 molecules helps the systems to maintain a constant value for Eint/CO2. The electron density data of non-covalent bond critical points in quantum theory of atoms in molecules (QTAIM) analysis are used to partition the total interaction energy data into primary anion⋯C and secondary O⋯C interactions. Furthermore, the multicenter charge delocalization in the anionic complexes is explained using the molecular electrostatic potential (MESP) analysis. This study proves that the anions possess a remarkable ability to interact with a large number of CO2 molecules due to cooperativity resulting from the secondary O⋯C interactions which compensate for the weakening of the primary anion⋯C interactions. This property of the anion–CO2 interactions can be exploited for developing anionic or anion-incorporated materials for CO2 storage.


 Please wait while we load your content...
Please wait while we load your content...