Probing the structure of giant fullerenes by high resolution trapped ion mobility spectrometry†
Abstract
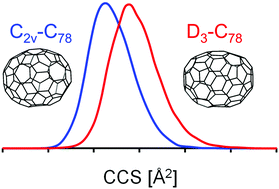
We present high-resolution trapped ion mobility spectrometry (TIMS) measurements for fullerene ions in molecular nitrogen. Three different charge states were studied (monocations, monoanions and dianions) with fullerenes ranging in size from C60 to C150. Ions were prepared by either electrospray ionization (ESI, for mono- and dianions) or by atmospheric pressure chemical ionization (APCI, for monocations) of a preformed fullerene soot extract solution. We demonstrate that TIMS allows to identify (and separate) constituent isomers in favorable cases. Using DFT calculations based on known condensed phase structures and trajectory method (TM) calculations we can reproduce the experimental TIMSCCSN2 for fullerenes up to C108 to within 0.5%. Using candidate structures based on quantum chemical predictions, we have also obtained structural information for fullerenes C110–C150 – a size range not previously accessed in condensed phase studies. We find that soluble fullerenes in this size have near-spherical rather than tubular structures. While the TM programs presently available for CCS modelling do a remarkably good job at describing the ion mobility of high (and even giant) fullerenes we observe a slight but systematic size-dependent deviation between TIMSCCSN2 values and our best computational fits which may reflect systematic bonding changes as the cage size increases.
- This article is part of the themed collections: PCCP Editor’s Choice, 2020 and 2019 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...