Cooperatively enhanced hydrogen bonds in ionic liquids: closing the loop with molecular mimics of hydroxy-functionalized cations†
Abstract
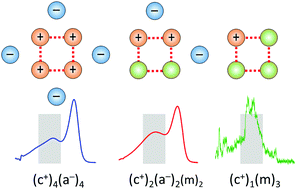
We address the cooperative hydrogen bonding interactions in play between the ionic constituents of ionic liquids (ILs) with particular attention to those involving the attractive interactions between two cations in the system 1-(2-hydroxyethyl)pyridinium tetrafluoroborate [HEPy][BF4]. This is accomplished by comparing the temperature-dependent linear infrared spectra of [HEPy][BF4] with that of the molecular mimic of its cation, 2-phenylethanol (PhenEthOH). We then explored the structural motifs of these H-bonded configurations at the molecular level by analyzing the cryogenic ion vibrational predissociation spectroscopy of cold (∼35 K) gas phase cluster ions with quantum chemical methods. The analysis of the OH stretching bands reveals the formation of the various binding motifs ranging from the common +OH⋯BF4− interaction in ion-pairs (c–a) to the unusual +OH⋯+OH interaction (c–c) in linear and cyclic, homodromic H-bonding domains. Replacing ion-pairs by the molecular (neutral) analogue of the IL cation also results in the formation of positively charged cyclic motifs, with the bands of the gas phase cationic cyclic tetramer (HEPy+)(PhenEthOH)3 appearing quite close to those assigned previously to cyclic tetramers in the liquid. These conclusions are supported by density functional theory (DFT) calculations of the cationic and neutral clusters as well as the local structures in the liquid. Our combined experimental and theoretical approach for the gas and the liquid phases provides important insight into the competition between differently H-bonded and charged constituents in liquids.
- This article is part of the themed collection: 2019 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...