A simple heuristic approach to estimate the thermochemistry of condensed-phase molecules based on the polarizable continuum model†
Abstract
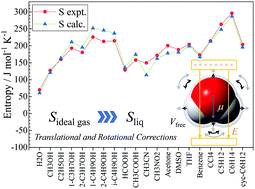
A simple model based on a quantum chemical approach with polarizable continuum models (PCMs) to provide reasonable translational and rotational entropies for liquid phase molecules was developed. A translational term was evaluated with free-volume compensation for the Sackur–Tetrode equation. We assumed that the free-volume corresponds to the cavity volume in the PCM. A rotational term was modeled as restricted rotation of a dipole in the electrostatic field. Entropies were assessed for twenty species in the liquid-phase using the proposed model, and the computed values were compared with experimental values. Quantum chemistry calculations were conducted at the ωB97X-D/6-311++G(d,p) level with the conductor-like PCM method. Predicted entropies were in good agreement with the experimental entropies, and the root mean square deviation was 17.2 J mol−1 K−1. The standard enthalpy change of formation was then investigated for eleven specific species. The CBS-QB3//ωB97X-D method provides a reasonable standard enthalpy of formation for gasified species; however, improvement of the accuracy is required for liquid species. Finally, the dependence of the Gibbs energy on temperature was investigated for the eleven specific species. When the ideal gas treatment is used, the Gibbs energy trends for the gaseous and liquid phases are quasi-parallel for all of the species, although the Gibbs energy trends for liquids based on the proposed model intersected the gaseous trend (i.e. the intersection is the boiling point). However, the model significantly under or overestimated the experimental boiling points. The error of the boiling points was predominantly due to the inaccuracy of the enthalpy.
- This article is part of the themed collection: 2019 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...