Nanotubols under H2O2 exposure: is it possible to poly-hydroxylate carbon nanotubes?†
Abstract
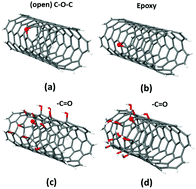
We present a combined experimental and theoretical study dedicated to analyze the variations in the surface chemistry of hydroxylated multiwalled carbon nanotubes (MWCNTs), so called nanotubols, when exposed to H2O2 at high temperatures. The formation, surface density, and distribution of oxygen-containing functional groups are studied by infrared (IR) and X-ray photoelectron spectroscopy (XPS), as well as density functional theory (DFT) calculations performed on model functionalized carbon nanotubes (CNTs). After H2O2 exposure, the initial composition of –OH, –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, and –COOH substituents notably changes, with carbonyl –C
O, and –COOH substituents notably changes, with carbonyl –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O groups being the ones that show the most notable increase on the carbon surface. Our highly oxidized MWCNTs are partially soluble and form complex two-dimensional patterns at the air–water interface, as evidenced by Brewster angle microscopy. In a second step, these films can be transferred to solid substrates to form porous multilayered carbon nanostructures with complex morphologies. In particular, and for the first time, we report the synthesis of “stadium-like” configurations made of MWCNT units whose formation and stability are a direct consequence of the self-assembly process occurring at the air/water interface. DFT calculations suggest the formation of molecular islands of oxygen-containing functional groups on the CNT surface. In addition, nudged elastic band studies reveal that, for these adsorbed phases, the reaction between two neighboring OH groups to produce atomic oxygen and a physisorbed water molecule is characterized by energy barriers of ∼0.2 eV. These small values could be at the origin of the sizable increase in chemisorbed single-oxygen species determined by XPS data after H2O2 treatment at 60 °C. The simulation of the C 1s binding energies (BE) allows us to more clearly identify the different oxygen-containing functionalities as well as to reveal how the local atomic environment affects their characteristic BEs. Even if we were unable to polyhydroxylate our carbon nanotubes, we believe that H2O2-treated MWCNTs are interesting materials for more complex post-functionalization procedures that might lead to the fabrication of novel carbon nanostructures.
O groups being the ones that show the most notable increase on the carbon surface. Our highly oxidized MWCNTs are partially soluble and form complex two-dimensional patterns at the air–water interface, as evidenced by Brewster angle microscopy. In a second step, these films can be transferred to solid substrates to form porous multilayered carbon nanostructures with complex morphologies. In particular, and for the first time, we report the synthesis of “stadium-like” configurations made of MWCNT units whose formation and stability are a direct consequence of the self-assembly process occurring at the air/water interface. DFT calculations suggest the formation of molecular islands of oxygen-containing functional groups on the CNT surface. In addition, nudged elastic band studies reveal that, for these adsorbed phases, the reaction between two neighboring OH groups to produce atomic oxygen and a physisorbed water molecule is characterized by energy barriers of ∼0.2 eV. These small values could be at the origin of the sizable increase in chemisorbed single-oxygen species determined by XPS data after H2O2 treatment at 60 °C. The simulation of the C 1s binding energies (BE) allows us to more clearly identify the different oxygen-containing functionalities as well as to reveal how the local atomic environment affects their characteristic BEs. Even if we were unable to polyhydroxylate our carbon nanotubes, we believe that H2O2-treated MWCNTs are interesting materials for more complex post-functionalization procedures that might lead to the fabrication of novel carbon nanostructures.


 Please wait while we load your content...
Please wait while we load your content...