Long-range electron–electron interaction and charge transfer in protein complexes: a numerical approach†
Abstract
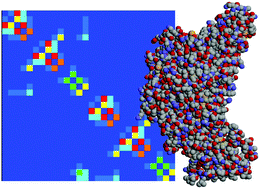
With application to the nitrite reductase hexameric protein complex of Desulfovibrio vulgaris, NrfH2A4, we suggest a strategy to compute the energy landscape of electron transfer in large systems of biochemical interest. For small complexes, the energy of all electronic configurations can be scanned completely on the level of a numerical solution of the Poisson–Boltzmann equation. In contrast, larger systems have to be treated using a pair approximation, which is verified here. Effective Coulomb interactions between neighbouring sites of excess electron localization may become as large as 200 meV, and they depend in a nontrivial manner on the intersite distance. We discuss the implications of strong Coulomb interactions on the thermodynamics and kinetics of charging and decharging a protein complex. Finally, we turn to the effect of embedding the system into a biomembrane.


 Please wait while we load your content...
Please wait while we load your content...