Tuning the electronic properties of the γ-Al2O3 surface by phosphorus doping†
Abstract
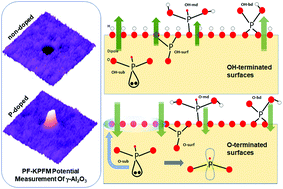
Tuning the electronic properties of oxide surfaces is of pivotal importance, because they find applicability in a variety of industrial processes, including catalysis. Currently, the industrial protocols for synthesizing oxide surfaces are limited to only partial control of the oxide's properties. This is because the ceramic processes result in complex morphologies and a priori unpredictable behavior of the products. While the bulk doping of alumina surfaces has been demonstrated to enhance their catalytic applications (i.e. hydrodesulphurization (HDS)), the fundamental understanding of this phenomenon and its effect at an atomic level remain unexplored. In our joint experimental and computational study, simulations based on Density Functional Theory (DFT), synthesis, and a variety of surface characterization techniques are exploited for the specific goal of understanding the structure–function relationship of phosphorus-doped γ-Al2O3 surfaces. Our theoretical calculations and experimental results agree in finding that P doping of γ-Al2O3 leads to a significant decrease in its work function. Our computational models show that this decrease is due to the formation of a new surface dipole, providing a clear picture of the effect of P doping at the surface of γ-Al2O3. In this study, we uncover a general paradigm for tuning support–catalyst interactions that involves electrostatic properties of doped γ-Al2O3 surface, specifically the surface dipole. Our findings open a new pathway for engineering the electronic properties of metal oxides’ surfaces.

 Please wait while we load your content...
Please wait while we load your content...